Unlike fresh fruits, fruit powder is made by advanced technologies so to extend the shelf life and versatility. We have Freeze Dried Fruit Powder and spray dried fruit powder. The star freeze dried powder are strawberry powder, raspberry powder, pink pitaya powder, blueberry powder and banana powder. Our fruit powder have no added sugar, sweeteners, preservatives,colors or flavorings which makes it perfect additive to food and beverage like ice cream, smoothie and sauce. Fruit powder promote bowel function and clean toxins, lower cholesterol absorption.
Fruit Powder,Mulberry Powder,Banana Powder,Apple Powder YT(Xi'an) Biochem Co., Ltd. , https://www.ytlinkherb.com
material 
Table 1 Ratio of transfection reagent volume to different DNA content. 

Figure 3 shows the results of uniform luminescence values. The firefly RLU was homogenized using the kelescence value of the sea kidney.
The SpectraMax iD5 Syringe Module and SmartInject technology oscillate during the addition of reagents to ensure that the reagents are fully mixed to maximize the fluorescent signal of fireflies and sea kidneys. 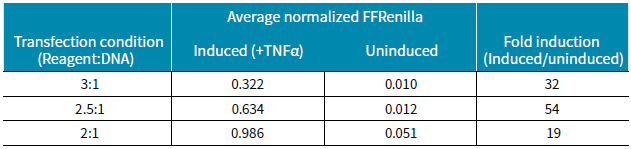
Detection of NF-κB activity using MD microplate reader and dual reporting system
Foreword
Reporter genes are a very useful tool for the study of gene expression. They can replace the gene of interest to be studied, thus helping us understand the signaling pathways and related diseases of the target gene. Luciferase is the most commonly used reporter gene, and we can easily detect luciferase using a photometric and chemiluminescent microplate reader. On the other hand, because of its low background in cell experiments, Has a high sensitivity. We usually use the level of firefly luciferase expression to reflect the status of the genes we are interested in in the system. Renin-derived luciferase is more commonly used as a second reporter gene in multi-fluorescence experiments to normalize a variety of indicators that vary widely in different samples, such as transfection efficiency and cell viability.
The SpectraMax® DuoLucTM Reporter kit provides highly sensitive quantification of firefly and Renilla luciferase in mammalian cells. Fluorescence can be detected by sequentially adding two appropriate amounts of reagents to the microplate wells. This dual fluorescent signal detection system provides a continuously expressed control fluorescent signal (renilla fluorescence) while normalizing the detection of fluorescent signals (firefly fluorescence). Experimental conditions can be optimized with the SpectraMax® iD5 Multi-Purpose Plate Reader, Syringe System and SmartInjectTM technology.
Here we show how to detect nuclear factor kappa B (NF-κB) activity in a mammalian cell model by a dual fluorescence reporter detection system and a SpectraMax iD5 microplate reader. NF-κB is a "primary regulator" involved in the expression of many genes in cellular processes such as inflammation, immunity, differentiation, proliferation and apoptosis. Tumor necrosis factor alpha (TNF-alpha) activates cellular signaling pathways to degrade NF-κB inhibitors, thereby promoting their release into the nucleus and regulating the expression of hundreds of genes. Disorders in the NF-κB pathway can induce a variety of diseases, such as multiple sclerosis, diabetes, Alzheimer's disease, etc., so it is necessary to use appropriate tools to deepen our understanding of the NF-κB pathway.
Advantage
• Sensitive dual reporting system for detection of multiple luciferase
• Normalization with control plasmid transfection to improve the accuracy of the results
• Preset templates for SoftMax Pro software simplify operation
Figure 1 NF-κB signaling pathway. The TNF-α activation signal cascade reaction degrades the NF-κB inhibitor IKB and releases the NF-κB transcription factor.
• SpectraMax Dual Reporting Kit (Molecular Devices cat. #R8361)
• HEK 293 cell line (ATCC cat. #CRL-1573)
• Medium: DMEM + 10% fetal bovine serum +penicillin/streptomycin
• pGL4.32[luc2P/NF-κB-RE/Hygro] Firefly luciferase plasmid (Promega cat. #E849A)
• pGL4.75[hRluc/CMV] Renilla Luciferase Plasmid (Promega cat. #E693A)
• FuGENE HD Transfection Kit (Promega cat. #E2311)
• Opti-MEM Low Serum Medium (ThermoFisher Scientific cat. #31985062)
• 96-well whiteboard (Corning cat. #3610)
• TNF-α 10 μg/mL PBS + 1 mg/mL BSA solution (Sigma cat. #T0157)
• BrightMax Sealing Film (Excel Scientific cat. #WT-50)
• SpectraMax iD5 multi-function microplate reader
• Syringe system with SmartInject technology
method
Cell seeding and transfection
HEK293 cells (80% confluency) were trypsinized and split into 96-well white plates at 15,000 cells per well. When the cells were more than 50% confluent, the cells were co-transfected with NF-κB-RE firefly luciferase and the sea kidney control plasmid, using three different ratios of FuGENE and DNA combination, then the cells were placed. Incubate in a 37 ° C incubator for 24 h.
TNF-α induction
A solution of 10 μg/mL TNF-α was diluted to 20 ng/ml with cell culture medium to prepare an induction solution. No added medium was used as a control. During the operation, the original medium was removed and 100 μL of the control or induction solution was added, after which the plate was returned to the incubator for further 7 hours.
Figure 2 Raw luminescence value data. The figure shows the RFU values ​​of TNF-α uninduced and induced under different transfection conditions (transfection reagent: DNA ratio). There are 9 replicates per transfection condition.
Preparation of cell lysate
After removing the cell culture medium, the cells in the well were washed twice with PBS. After the lysis buffer was returned to room temperature, 20 μL was added to each well. Gentle shaking for 15 minutes at room temperature promotes cell lysis. A whiteboard sealing cap is placed on the bottom of the plate to enhance the fluorescence signal intensity.
Luciferase assay setting
The reagents in the kit should be incubated at room temperature before use. The firefly enzyme substrate was prepared by adding 220 μL of water to a vial containing 2.2 mg of the lyophilized substrate. To the vial containing 440 μg of the lyophilized substrate, 220 μL of water was added and mixed to obtain hirudin. The firefly working solution is prepared by diluting the firefly enzyme substrate with a firefly detection buffer at a ratio of 1:50. The sea kidney working solution was prepared by diluting hirudin with a ratio of 1:50 in Renilla luciferase buffer. For a 96-well plate, we need 11 mL of each working solution, which contains 220 μL of each substrate. Pre-configured programs in SoftMax® Pro Software help perform experimental and result analysis, which requires the following parameter settings:
1. Add 100 μL of firefly working fluid to the well using syringe 1
2, wait 2 seconds to make the reaction full
3, set the integration time 5 seconds to detect firefly fluorescence
4. Add 100 μL of sea kidney working solution to the well using syringe 2.
5, wait 2 seconds to make the reaction sufficient
6, set the integration time 5 seconds to detect the Renilla fluorescence
7. For each experimental well, the data is obtained by comparing the first measurement (firefly fluorescence) with the second measurement (sea kidney fluorescence).
Table 2 Ratios obtained under different transfection conditions with and without induction. The best condition is transfection reagent: DNA is 2.5:1.
result
The high expression of Renilla luciferase was detected by various transfection conditions with respect to changes in induction conditions, and the addition of the cytokine TNF-α greatly increased the expression level of firefly luciferase (Fig. 2). In summary, the expression level of luciferase was lowest at the ratio of transfection reagent to DNA of 2:1 compared to other ratios. The results showed that under the conditions of induction, the homogenization of firefly luciferase and Renilla luciferase was higher under the three transfection ratios (Fig. 3). However, the homogenization ratio was lower under the transfection conditions of 3:1 and 2.5:1, but the relationship between the induction of TNF-α and the undoped ratio was significantly greater. Table 2 shows the fold ratios induced under the three transfection conditions. The best induction ratio is the reagent:DNA ratio of 2.5:1.
in conclusion
Using the double reporter gene assay in the HEK293 cell line, we demonstrated that the addition of TNF-α strongly induced the expression of NF-κB. Since this system is sensitive to both firefly luciferase and Renilla luciferase, a stronger signal can be obtained. Under the three transfection conditions, the optimal ratio of NF-κB induced by TNF-α was 54 times.
Use the SpectraMax® iD5 Multi-Purpose Plate Reader, a SmartInjectTM technology injector system for testing SpectraMax® DuoLucTM Reporter experiments. The preset operational flow simplifies the operation of data acquisition and analysis. SpectraMax Pro software helps you get the results of a dual luciferase reporter system quickly, completely, sensitively and reliably.
Reference
1. Oeckinghaus, A and Ghosh, S. The NF-κB Family of Transcription Factors and Its Regulation.
Cold Spring Harb Perspect Biol. 2009 Oct; 1(4): a000034.