Cosmetic raw material powder
Xi'an Jmlai Bio-Tech Co.,Ltd is Research, Production, Process and Sales. company mainly engaged in export of products including,without limitation, pharmaceutical raw materials both for vet and human, also Vitamins, Amino Acids, minerals, peptides,plantextract, feed and food additives. and provide natural and well-qualified raw materials to the companies being engaged in pharmaceutical,health product, nutritional product, cosmetic raw material,Sarms Products , Steroids Products Peptides Products, APIProducts, Plant Extract and HGH, HCG. with very strong strength in R&D, production, sale and other aspects in the national plant extract industry.
Cooper Peptide,Cosmetic Raw Material,Kojic Acid,Alpha-Arbutin Xi'an Jmlai Bio-Tech Co., Ltd. , https://www.jmlaisarms.com
In order to reasonably protect Cordyceps sinensis resources, we have effectively developed and utilized Cordyceps sinensis resources, promoted research and development of high-end scientific and technological content of health foods, and researched and established effective supervision methods for the use of rare and valuable raw materials for health foods, in accordance with the “Food Safety Law of the People's Republic of China†and “China The People's Republic of Wild Plant Protection Regulations and other laws and regulations, the State Food and Drug Administration has organized the "Cattle Cordyceps sinensis for health food pilot program." The food and drug supervision and administration departments of all provinces (autonomous regions and municipalities) shall, in accordance with the actual conditions of the health food production enterprises within their respective administrative regions, organize the relevant pilot work according to the requirements of the pilot work plan.
The State Food and Drug Administration August 15, 2012 Cordyceps sinensis for health food pilot work program To properly carry out the pilot work of Cordyceps sinensis for health foods, research and establish effective supervision methods for the use of rare resources for health foods, and formulate this pilot work program.
I. Purpose of work Through this pilot work, scientific and strict establishment of entry barriers, and actively guide qualified health food production enterprises under the premise of reasonable protection of Cordyceps sinensis resources, efficient development and utilization of Cordyceps sinensis resources, and promote the development of high-end scientific and technological content of health food To promote the improvement of the quality of food safety and control. At the same time, it lays the foundation for establishing a regulatory model for the use of rare resources for health foods.
II. Work Principles (1) Work is carried out according to law. In strict accordance with the "Regulations of the People's Republic of China on the Protection of Wild Plants" promulgated by the State Council, "Regulations on the Collection and Management of Licorice and Ephedra" and Regulations of the Ministry of Agriculture and "Replied to the General Office of the Ministry of Health on Cordyceps as a Raw Material for Health Food" The Office of Supervision (2012) No. 39) asked for related work.
(B) the rational use of rare resources. Strengthen the controllability of Cordyceps sinensis resources, strictly monitor the source of Cordyceps sinensis raw materials, strengthen the supervision of the whole process of processing and use, and protect Cordyceps sinensis resources and grassland ecological environment.
(c) strict access standards. Strict conditions for the entry of pilot enterprises were formulated, relevant requirements were clarified, high-level R&D and deep processing of Cordyceps sinensis was emphasized, and the scientific and rational development and utilization of limited resources of Cordyceps sinensis was realized.
III. Basic conditions of pilot enterprises (1) Sources of stable and legal Cordyceps sinensis. It is able to provide purchase and sales contracts that are legally signed by the relevant provinces of the resource provinces and can guarantee large-scale production. The scale of product production is compatible with the Cordyceps sinensis resources obtained, which can ensure the sustainability of production.
(B) have a good health food research and development capabilities. In the past two years, the pilot companies have undertaken national-level technical research in the food and drug field or major national science and technology innovation projects. They have a clear innovation and development strategy and a research and development team, and have good research and development equipment, laboratories and other conditions and capabilities, and obtained national technology patents.
(C) has significant technical advantages. Based on the trial production of Cordyceps sinensis as a raw material, a large number of basic research and verification work have been carried out, and there is sufficient scientific basis for support (literature basis, authoritative experts' approval), and the researched and developed products have potential for market development.
(D) have a good health food production capacity. The annual sales volume of pilot enterprises’ health food industrial products is more than one billion yuan (the production enterprises of health foods at the place of origin can be appropriately relaxed). With advanced production level and complete inspection and inspection capabilities, it has good product quality and safety process control capabilities.
(five) have the industry leading. The pilot companies' market share in the product ranks among the top in the country, with a greater influence, and has a well-known Chinese trademark. Pilot enterprises should have good social recognition and influence, honesty and trustworthiness, strong sense of social responsibility, no relevant negative reports on quality and safety within two years, no major quality and safety liability accidents, no fake sales records, and good operating conditions.
IV. Pilot Contents (1) Specification of Cordyceps sinensis raw material procurement management. To study the management methods of Cordyceps sinensis procurement, strictly control the source of Cordyceps sinensis, study the technical requirements and corresponding detection methods of Cordyceps sinensis raw materials, and strictly control the quality of Cordyceps sinensis raw materials.
(B) improve product quality and safety control level. Strengthen the application of high-tech means, strict product quality safety and security measures, strengthen the whole process of management, and explore effective ways to establish product quality and safety process control. Establish a traceability system for the whole process of production and operation that guarantees product quality and safety, covering the entire process of raw material procurement, feeding, intermediate control, finished product inspection, and sales.
(3) Conduct post-marketing product efficacy monitoring and verification. Formulate measures for the follow-up monitoring of the efficacy of sold products, accumulate evaluation data, complete evaluation reports, and study effective methods for establishing product efficacy verification.
V. Pilot work steps (1) Within one year from the date of issuance of the relevant notice of the State Food and Drug Administration, the enterprise shall, within the prescribed time, submit a pilot application to the provincial food and drug regulatory authority at the place where the manufacturer is located according to the basic conditions of the pilot enterprise. Submit relevant information.
(2) After the provincial food and drug regulatory authority has accepted the enterprise's application, it will review the relevant materials and submit an audit opinion. If it meets the requirements, it shall be submitted to the State Food and Drug Administration.
(3) The State Food and Drug Administration organizes the review of relevant materials submitted by the provincial food and drug regulatory authorities. If they meet the requirements, they agree that the enterprises participate in the pilot work and give feedback to the relevant provincial food and drug regulatory authorities.
(4) Relevant enterprises shall carry out product R&D and relevant test verification in accordance with the product registration application procedures and related requirements, prepare relevant application materials, and submit product registration applications. The State Food and Drug Administration has accelerated the review, approval and approval of products in accordance with the principle of “no reduction in procedures and no reduction in standardsâ€. If it meets the requirements, it is allowed to register and issue a health food approval certificate. If the health food approval certificate is based on the substitute of Cordyceps sinensis, the applicant may carry out relevant declaration work in accordance with the change procedures and relevant requirements.
(5) Relevant enterprises organize product production according to the contents approved by the State Food and Drug Administration, and submit a pilot work report to the State Food and Drug Administration in accordance with the contents of the pilots at the end of each year of product approval. At the same time, it is also necessary to submit proof of raw materials for the purchase of Cordyceps sinensis, use flow of Cordyceps sinensis, and sales of related products.
(6) The State Food and Drug Administration organizes the review of the pilot work report provided by the company. In compliance with the requirements, related companies continue to carry out pilot work. If it does not meet the requirements, the State Food and Drug Administration may require relevant companies to stop the pilot work and write off the relevant health food approval number.
(7) During the pilot period, if the pilot company has any negative reports on product quality and safety, or any major quality and safety liability accidents or any counterfeiting or counterfeiting activities, the SFDA will order the relevant companies to stop the pilot work once it is verified. Write off the relevant health food approval number and deal with it severely according to law.
6. The pilot time limit for the pilot period is five years from the date of approval of the relevant products of the pilot enterprises by the State Food and Drug Administration. 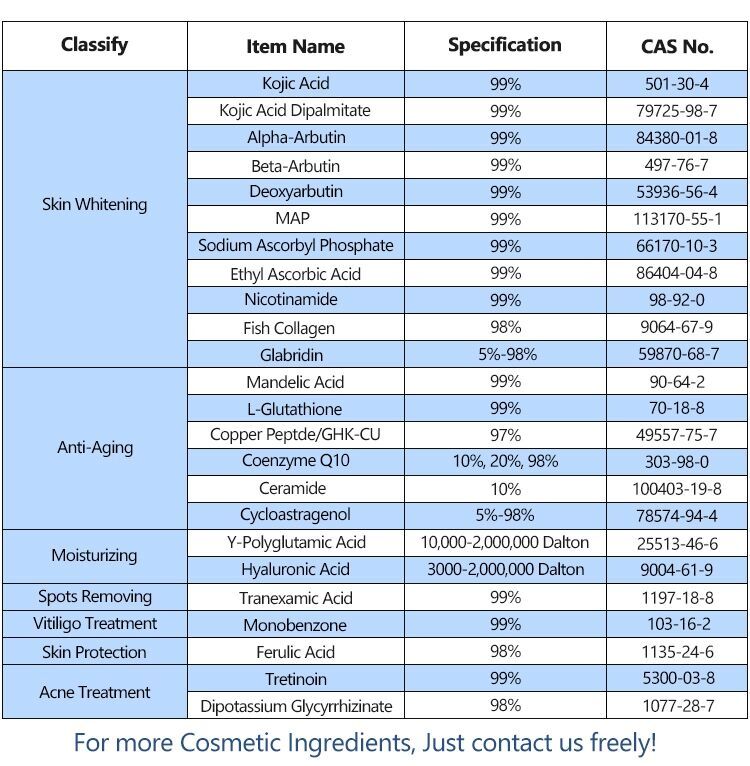
Notice on Printed Cordyceps Sinensis Used in Health Food Pilot Program
Food and Drug Administration (Drugs and Drugs Supervision Bureaus) of provinces, autonomous regions, and municipalities directly under the Central Government, Health Food Evaluation Center of the State Food and Drug Administration:
Company Profile